PET and PBT: Crystallization makes the difference
May 29, 2024, 11:58 AM
TDD-global
6440
The chemical structures of PET and PBT are basically very similar. Polyester is synthesized from an organic acid (here, terephthalic acid) and an alcohol. For PBT, its alcohol monomer becomes butanediol, while PET is ethylene glycol. The polymers thus obtained are well known - PBT and PET.
The chemical structures of PET and PBT are basically very similar. Polyester is synthesized from an organic acid (here, terephthalic acid) and an alcohol. For PBT, its alcohol monomer becomes butanediol, while PET is ethylene glycol. The polymers thus obtained are well known - PBT and PET.
The best way to understand the difference between PBT and PET is to examine the chemical structure of the repeating units that make up the polymer molecular chain, as shown in Figure (1). The basic feature that sets PBT and PET apart from other materials is the terephthalate group, from which the name of this class of materials comes from. Other polymers, such as PTT and PCT, are also members of this chemical family, showing only slight differences in their structures.
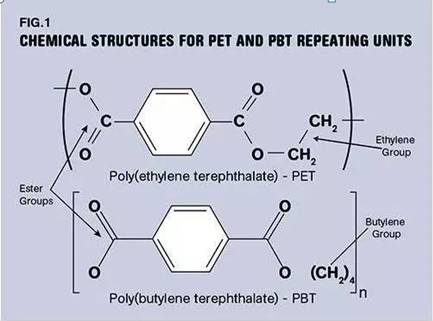
Another key chemical feature of this class of materials is the presence of six-membered rings at regular intervals on the polymer backbone. This structural unit called a benzene ring, also commonly known as an aromatic ring, provides rigidity to the polymer chain. This will affect several important properties, including the glass transition temperature, above which the polymer loses most of its load-carrying properties.
The 2D perspective of the chemical structure is not very obvious, but the 3D perspective shows that the aromatic rings lie within a bright plane, regardless of whether many chemical groups on the polymer chain can or cannot be projected onto a paper plane. Aromatic rings therefore limit the intrinsic properties of rotation and vibration of other groups on the molecular chain. This is part of the hardening effect of the aromatic ring structure. This reduced mobility of the benzene ring and its bulky nature will also affect the ability of the polymer to crystallize on cooling. PBT has a larger spacing of aromatic benzene rings, and the crystallization is more efficient than PET. However, PET, if crystallized more efficiently, would provide better mechanical properties, including strength, stiffness and performance at high temperatures.
Many consumers are familiar with PET materials for containers for bottling water and soft drinks. This type of PET is amorphous and engineered to prevent it from crystallizing. If the PET grade for the bottle crystallizes, it becomes cloudy and opaque, and more importantly, it loses its impact resistance. So, while there may be many parts under the hood that are molded from crystalline PET, there are high temperatures and harsh chemical environments; and most of the PET in the world is consumed in packaging, where it is amorphous, non-crystalline reinforced , cannot cope with the above harsh environment.
The PET types we will discuss in the second part of this article are amorphous and always contain a high proportion of glass fibers and/or mineral fillers. However, polyester PBT is provided in its crystalline form, either filled or unfilled. In fact, since PBT crystallizes faster than PET, it is impossible to produce amorphous PBT parts under normal processing conditions. If polymers are crystallized efficiently enough, they can all achieve a certain level of organization in their structure. The rigidity of the polyester group and aromatic ring is balanced with the flexibility and mobility of the butyl group. However, in PET, the shorter vinyl groups allow for selectivity in crystallization. If we cool PET quickly, we get amorphous PET; otherwise, we cool it slowly, and we get semi-crystalline PET.
Many PET bottles start out as preforms by injection molding. These preforms are transparent with thicker walls in order to prevent the thinning effect experienced when subsequent preforms are reheated and stretched into bottles. If you've ever worked in a bottle factory, you know that during the preheating stage, if the preforms get too hot, they will become cloudy and opaque - a sign of crystallization. (In fact, if you get close to the gate of the preform, you will see a small amount of fog in this area, caused by the part overheating in this part). If you were trying to blow mould a bottle with this opaque partially crystallized preform, that would reduce impact performance. If enough crystals are formed, the preform may even break during the blow molding process. So, the trick is to keep the material above its glass transition temperature and below its crystallization temperature. This temperature window may not be very wide, as shown in Figure (2).
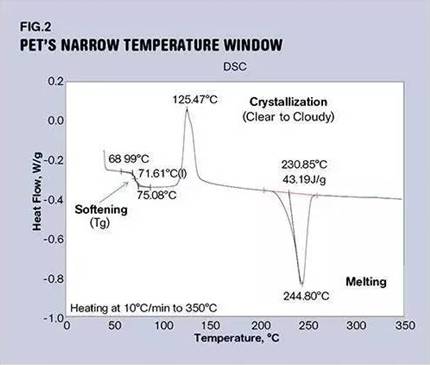
This graph shows the behavior of amorphous PET polyester, an unfilled clear material used to make parts that require toughness and clarity but not high temperature performance. When the material is heated from room temperature, the first notable phenomenon is the glass transition. This appears to be a step in the enthalpy change of the material in which the process is done at 75°C (167F). At this point, the material loses its rigidity at room temperature and becomes soft and compliant. As the temperature increased, the viscosity of the softened polymer began to decrease until it approached 110°C (230F). This is the temperature at which the scan baseline begins to rapidly increase, and the gap between 75°C and 110°C represents the window of opportunity for blow molding PET bottles.
Many bottling plants I've visited use preheat temperatures close to 100C (212F). Once the crystallization process kicks in, the material starts to blur. The material will then begin to regain some of the stiffness it lost as it passed the glass transition temperature. If this process continues, the polymer will crystallize at 140°C (473F). Therefore, PET can exist in two forms - amorphous or semi-crystalline - depending on how it is handled.
However, under normal commercial conditions, PBT is always semi-crystalline. So, to properly understand the performance difference between PET and PBT, we need to compare the crystal formation of each polymer against a reasonable standard. Because PET crystallizes very slowly, the use of materials with this semi-crystalline structure to make parts requires the help of certain chemicals, such as nucleating agents, and some solid particles, such as fillers and reinforcing agents. Therefore, commercial semi-crystalline PET is always solid filled or reinforced. In order to make a fair comparison of the performance of PET and PBT, we therefore need to compare materials of the same type of filler with the same filling ratio.
The best way to understand the difference between PBT and PET is to examine the chemical structure of the repeating units that make up the polymer molecular chain, as shown in Figure (1). The basic feature that sets PBT and PET apart from other materials is the terephthalate group, from which the name of this class of materials comes from. Other polymers, such as PTT and PCT, are also members of this chemical family, showing only slight differences in their structures.

Another key chemical feature of this class of materials is the presence of six-membered rings at regular intervals on the polymer backbone. This structural unit called a benzene ring, also commonly known as an aromatic ring, provides rigidity to the polymer chain. This will affect several important properties, including the glass transition temperature, above which the polymer loses most of its load-carrying properties.
The 2D perspective of the chemical structure is not very obvious, but the 3D perspective shows that the aromatic rings lie within a bright plane, regardless of whether many chemical groups on the polymer chain can or cannot be projected onto a paper plane. Aromatic rings therefore limit the intrinsic properties of rotation and vibration of other groups on the molecular chain. This is part of the hardening effect of the aromatic ring structure. This reduced mobility of the benzene ring and its bulky nature will also affect the ability of the polymer to crystallize on cooling. PBT has a larger spacing of aromatic benzene rings, and the crystallization is more efficient than PET. However, PET, if crystallized more efficiently, would provide better mechanical properties, including strength, stiffness and performance at high temperatures.
Many consumers are familiar with PET materials for containers for bottling water and soft drinks. This type of PET is amorphous and engineered to prevent it from crystallizing. If the PET grade for the bottle crystallizes, it becomes cloudy and opaque, and more importantly, it loses its impact resistance. So, while there may be many parts under the hood that are molded from crystalline PET, there are high temperatures and harsh chemical environments; and most of the PET in the world is consumed in packaging, where it is amorphous, non-crystalline reinforced , cannot cope with the above harsh environment.
The PET types we will discuss in the second part of this article are amorphous and always contain a high proportion of glass fibers and/or mineral fillers. However, polyester PBT is provided in its crystalline form, either filled or unfilled. In fact, since PBT crystallizes faster than PET, it is impossible to produce amorphous PBT parts under normal processing conditions. If polymers are crystallized efficiently enough, they can all achieve a certain level of organization in their structure. The rigidity of the polyester group and aromatic ring is balanced with the flexibility and mobility of the butyl group. However, in PET, the shorter vinyl groups allow for selectivity in crystallization. If we cool PET quickly, we get amorphous PET; otherwise, we cool it slowly, and we get semi-crystalline PET.
Many PET bottles start out as preforms by injection molding. These preforms are transparent with thicker walls in order to prevent the thinning effect experienced when subsequent preforms are reheated and stretched into bottles. If you've ever worked in a bottle factory, you know that during the preheating stage, if the preforms get too hot, they will become cloudy and opaque - a sign of crystallization. (In fact, if you get close to the gate of the preform, you will see a small amount of fog in this area, caused by the part overheating in this part). If you were trying to blow mould a bottle with this opaque partially crystallized preform, that would reduce impact performance. If enough crystals are formed, the preform may even break during the blow molding process. So, the trick is to keep the material above its glass transition temperature and below its crystallization temperature. This temperature window may not be very wide, as shown in Figure (2).

This graph shows the behavior of amorphous PET polyester, an unfilled clear material used to make parts that require toughness and clarity but not high temperature performance. When the material is heated from room temperature, the first notable phenomenon is the glass transition. This appears to be a step in the enthalpy change of the material in which the process is done at 75°C (167F). At this point, the material loses its rigidity at room temperature and becomes soft and compliant. As the temperature increased, the viscosity of the softened polymer began to decrease until it approached 110°C (230F). This is the temperature at which the scan baseline begins to rapidly increase, and the gap between 75°C and 110°C represents the window of opportunity for blow molding PET bottles.
Many bottling plants I've visited use preheat temperatures close to 100C (212F). Once the crystallization process kicks in, the material starts to blur. The material will then begin to regain some of the stiffness it lost as it passed the glass transition temperature. If this process continues, the polymer will crystallize at 140°C (473F). Therefore, PET can exist in two forms - amorphous or semi-crystalline - depending on how it is handled.
However, under normal commercial conditions, PBT is always semi-crystalline. So, to properly understand the performance difference between PET and PBT, we need to compare the crystal formation of each polymer against a reasonable standard. Because PET crystallizes very slowly, the use of materials with this semi-crystalline structure to make parts requires the help of certain chemicals, such as nucleating agents, and some solid particles, such as fillers and reinforcing agents. Therefore, commercial semi-crystalline PET is always solid filled or reinforced. In order to make a fair comparison of the performance of PET and PBT, we therefore need to compare materials of the same type of filler with the same filling ratio.
August 21, 2024, 2:42 PM
August 21, 2024, 2:28 PM
August 21, 2024, 2:20 PM
August 21, 2024, 3:01 PM